Märtsis 28, the us food and drug Administration (FDA) introduced a new “emergency-use-administration” (EUA), stating that there are only six countries and regions with acceptable standards for masks that meet specified performance standards. The Chinese mask standards that were recognized two weeks ago disappeared from the list!
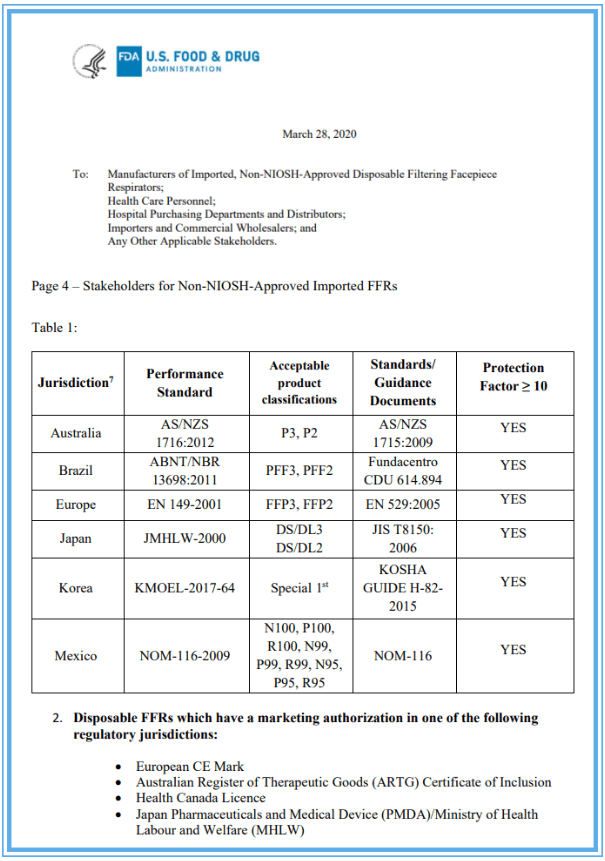
Märtsis 28, the US FDA updated the Non-NIOSH approved emergency use permit EUA. In this published document, it is clearly mentioned that the respirator products provided by other countries have been evaluated using some methods similar to NIOSH. According to the guidance issued by the US FDA, Chinese KN95 masks are no longer approved for use in the US. The guide excludes China from the Non-NIOSH country list. Masks made in China including KN95, KP100, KN100 and KP95 will not be listed on the EUA list.
On March 17th, two weeks ago, the US Centers for Disease Control and Prevention (CDC) announced the “Strategy for Optimizing the Supply of N95 Respirators: Crisis / Replacement Strategy”, and approved that other countries’ masks of the same level as N95 can be used in the United States. , The list contains Brazil, South Korea, Japan, Australia, Europe, Mexico and China (including four domestic mask models: KN100, KP100, KN95, KP95), a total of seven countries and regions. Why did the FDA, which previously claimed to accept the Chinese mask standard, disappear after only two weeks? Moreover, comparing the two notices successively, China is the only country in the list of 7 countries that are going to be approved for standards, except China!
Previously, after undergoing rigorous testing, the CDC of the US Centers for Disease Control recognized China ’s mask standards and technical standards, and stated that KN95 masks are one of many “suitable alternatives” to N95 masks in short supply. Siiski, the specifications that KN95 complies with are slightly different from those of N95, and have not been certified by the US government.
It is understood that the KN95 mask has a filtration rate of at least 95% for non-oily particles with a size of 0.3 microns or larger, which has the same effect as N95. Allowing the import and use of KN95 will greatly alleviate the shortage of masks in the United States, but without FDA approval, importers would not dare to order KN95 masks because they fear they will be detained by customs. According to industry sources, the newly approved masks from the United States from Australia, Brazil, Japan, South Korea, Mexico and the European Union have far less capacity than N95 or KN95. The FDA’s decision caused a lot of doubts from the outside world, but at present, FDA has not made any comments on the outside world’s doubts.
According to the announcement, when NIOSH became aware of counterfeit masks or distorted NIOSH approved masks in the market, the US CDC issued a list of fake masks to remind users, buyers and manufacturers.
 VIGA segisti tootja
VIGA segisti tootja 
